Super poo: the emerging science of stool transplants and designer gut bacteria |
Good poo donors are so hard to find they’re sometimes called “unicorns”. These elusive, healthy creatures service a market for faecal transplants that is growing rapidly as evidence of its benefits mounts.
Emerging science shows that a human’s microbiome – their constellation of gut microbes – has a far greater effect on health than anyone previously imagined. This enormous ecosystem we host in our bodies includes bacteria, fungi, viruses and more.
The collective genetic material in the microbiome performs myriad functions that affect our mood, our immunity, and our physical and mental health.
Crappy western diets and antibiotics are depleting our microbiota. And in some cases, a person’s microbiome is disordered enough that it needs a little boost from someone else’s.
Restoring them to health has become a very serious scientific endeavour, as the diversity of our gut bacteria is linked to everything from depression to how we respond to cancer treatment.
The transfer of healthy stool into the gastrointestinal tract of an unhealthy recipient has been proven to treat people with intestinal conditions, including the superbug Clostridium difficile colitis, or C diff – which can cause diarrhoea, sepsis and even death.
But as scientific understanding of the microbiome improves, the possibilities of faecal transplants are expanding.
Now researchers are working on a “super stool” – a poo pellet you can eat that mimics a so-called unicorn’s special abilities.
Replicating unicorns
BiomeBank’s office is located in a hub in the suburbs of Adelaide. Chief medical officer Sam Costello and chief executive Thomas Mitchell wear lanyards emblazoned with depictions of bacteria – and Costello jokes that an organic-looking spill on his is probably a real-life experiment.
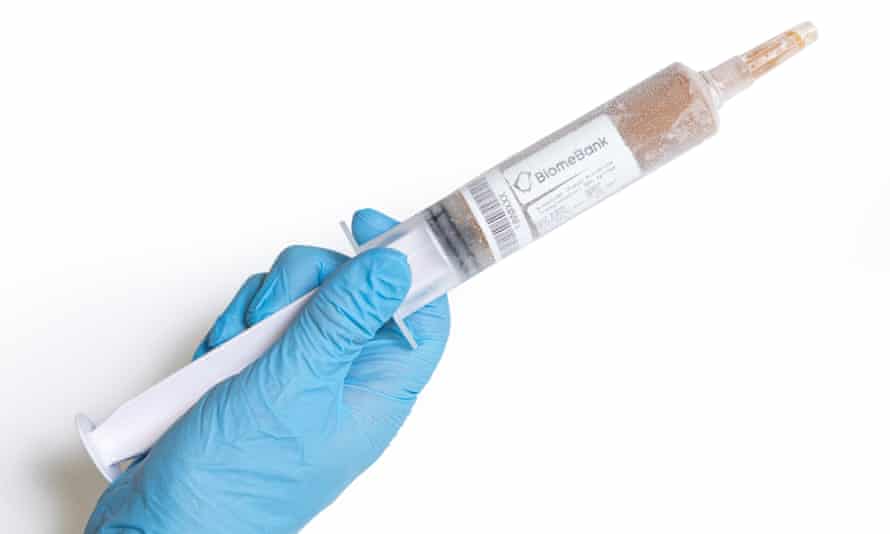
The biotech company has been on a unicorn hunt. This place is home to a stool bank – and it’s a place where designer poo transplants are made.

There’s a special donor room (a glorified toilet) where poo donations are received. There’s a lab with an anaerobic work station, where donors’ bacteria strains are put into a “secret sauce” to grow and then isolate strains. Those strains are categorised and catalogued for future use.
BiomeBank’s head of donor screening, Dr Emily Tucker, says there’s a long list of requirements for a stool donor. They have to be healthy, obviously. They have to be screened for infections. A detailed history of their medical, travel and antibiotic history is taken.
Those who make it through all the assessments are enrolled for an eight-week program where they have to turn up on time, fill out a questionnaire, then (ahem) make a deposit inside a special room.
So what happens if there aren’t enough unicorns? That question inspired BiomeBank’s latest endeavour – to replicate the contents of a unicorn’s guts.
The poo factory
People are building libraries of the very best that poo has to offer, and BiomeBank is part of the effort to categorise premium stool strains.
Costello, a gastroenterologist, says that humans historically had much more diverse microbiota. We lived more closely with other people and with animals, and we ate more unprocessed food.
There’s evidence, he says, that this depletion is evident across society – particularly western society.
He describes a “microbial extinction event” in the modern world. As on Earth, so it is in our intestinal system – we’re living with the consequences of a hollowed-out ecosystem.
Mitchell explains that in BiomeBank’s first generation of microbial therapy they extracted the right bacteria, freeze dried it and put it in a capsule.
Patients can take it orally to treat specific infections. This generation has already been rolled out in hospitals, and, if approved by the Therapeutic Goods Administration, it will be the first microbial therapy in the world approved as a biologic (it currently has provisional approval).
Then there’s the second generation – a replication. Think of the unicorns as Adam and Eve types, but their genomic sequences can be reproduced. You can grow them. Isolate them. Identify them, name them, put them in a library, and gradually build up knowledge about what each strand can do.
And when you hit a vein of gold – a bacterial strain that sorts out a deficiency in someone’s microbiota – you scale it. Then you put it that bespoke bacterial recipe in a capsule that someone else swallows. “It’s a new way to treat disease,” Mitchell says. “It’s a little factory.”
Why is the microbiome so important?
From the moment we’re born, and throughout our lives, our microbiota are shaped by our environmental and dietary inputs.
Babies born by caesarean section have a different mix to those delivered vaginally, a…
Read More: Super poo: the emerging science of stool transplants and designer gut bacteria |

