Vaxart: Too Early To Dismiss This COVID-19 Vaccine Opportunity (NASDAQ:VXRT)
Investment Thesis
The stock prices of biotechs developing vaccine solutions for COVID-19 have declined markedly over the past couple of weeks, despite the need for a coronavirus cure being greater than ever.
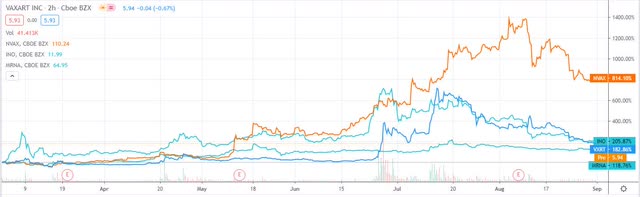
Performance of selected biotech vaccine/adjuvant developers Vaxart, Moderna, Novavax, and Invio, past 6 months. Source: TradingView.
Although many COVID-19 vaccine or adjuvant developers still trade hundreds of percentage points above their pre-pandemic levels, as we can see above, it appears the market has become desensitised to the steady stream of news flow relating to manufacturing and distribution deals, early-stage trial data and novel treatment types, and is now laser-focused on cold, hard data – the later the trial stage, the better.
Vaxart (VXRT) is one vaccine developer that has come in for particularly stinging criticism in recent weeks, but despite this, the microcap, in my view, is probably more valuable now than at any time in its history.
The initial enthusiasm for the company’s solution – the only currently credible oral COVID-19 vaccine currently in development, which saw Vaxart’s stock price climb from $0.35 in January, to a high of $17 in mid-July, has faded, for two main reasons, in my view.
Firstly, due to the company’s relatively slow progress in comparison to better-resourced rivals (Vaxart had only 16 full-time employees and $44m of cash as at Q220 and has yet to enter a phase 1 trial), and secondly, due to the perception that Vaxart misrepresented its progress by reporting it had been selected to participate in the US Government’s Operation Warp Speed programme (“OWS”), which aims to have an approved vaccine on the market by January 2021 when, in reality, it had merely entered its vaccine into a non-human primate trial organised by OWS.
The “news” caused Vaxart’s stock price to sky-rocket to $17, allowing the company’s one-time majority shareholder Armistice Capital to reportedly make a $200m profit selling shares and warrants – that it was able to exercise at prices of $0.3 and $1.1 – in bulk, in June and July, offloading nearly its entire holding in the company. This may even have been facilitated by Vaxart – whose newly appointed CEO and President Andrei Floroiu had a lengthy prior working relationship with Armistice – changing the terms of the warrants in the weeks prior to the sales.
Arguably, however, Vaxart has done nothing wrong. Armistice came to the company’s rescue when it was desperate for funds, and as per the rules of capitalism, made a significant profit when circumstances conspired to make vaccine developers the hottest shares on the market.
Additionally, Vaxart was able to raise $97m via a share offering at a price of $6.78, which gives the company a fighting chance of competing for the lucrative prize of being, not necessarily the first, but possibly one of the most effective, convenient and environmentally friendly COVID-19 vaccine solutions on the market.
I do not think Vaxart’s recent share price decline – to $6.23 at the time of writing – suggests that the company’s vaccine race is run. Vaxart submitted an Investigational New Drug (“IND”) application to the FDA on August 10th to begin a phase 1 trial of its COVID-19 vaccine candidate, and as I will discuss shortly, although it is undoubtedly a long shot for approval, the fact the company’s candidate is orally administered gives it a significant competitive advantage over the rest of the field – if it can deliver on the efficacy front.
I don’t subscribe to the view that the first vaccine candidate to be approved will decimate the share prices of all of its drug-development rivals since all candidates have different strengths and weaknesses, which suggests the race to find the best solution will continue for at least another 12-18 months, with plenty more ups and downs and surprises.
As such, I expect there to be significant further volatility in vaccine stocks going forward and based on its differentiated approach, bolstered cash resources, and encouraging albeit limited data sets, I believe Vaxart’s shares will rise again. Thankfully, the downside case is limited by the fact that Vaxart has 2 other vaccine candidates ready to move into phase 2 testing and has an agreement in principle to develop vaccine candidates for Janssen, a subsidiary of Johnson & Johnson, which could provide a long-term income stream for the company, as well as retail and institutional investment.
Analysts have set a consensus price target of $19.5 for Vaxart stock. I see the company’s upside potential as practically unlimited given what is at stake, but more realistically, a promising phase 1 data set, faster than expected progress towards a larger scale trial, or a partnership deal of real significance (possibly even an acquisition) can catapult Vaxart’s stock price back to and beyond its July high of $17 almost overnight, which is why I still make Vaxart a buy and have added it to my model biotech portfolio at my…
Read More: Vaxart: Too Early To Dismiss This COVID-19 Vaccine Opportunity (NASDAQ:VXRT)